I. Why do isotopes fractionate ?
Isotope fractionation is characterized by a difference in reactivity of isotopes during a chemical reaction resulting in a different isotopic ratio between product and reactant. These fractionations are caused by numerous processes (change in degree of oxidation, adsorption, evaporation, etc.) and are often complex to apprehend, describe or predict. The study of variations in isotopic compositions of metal contaminants between different environmental compartments can provide information on the origin of these contaminants and/or on the processes that concern them during their transport.
In other words, we can see the atomic bonds between the different elements as springs. The robustness of these springs will depend, among other things, on the length of the bond and the mass of the bonded atoms. The heavier the atoms, the more likely the atomic bonds will be to break. In fact, not all atomic bonds are equal and these differences can therefore lead to isotopic fractionations, i. e. isotopic signature changes by preferentially concentrating heavy or light isotopes in a phase or during a chemical reaction.
In this lesson, we will only speak about mass dependant fractionation.
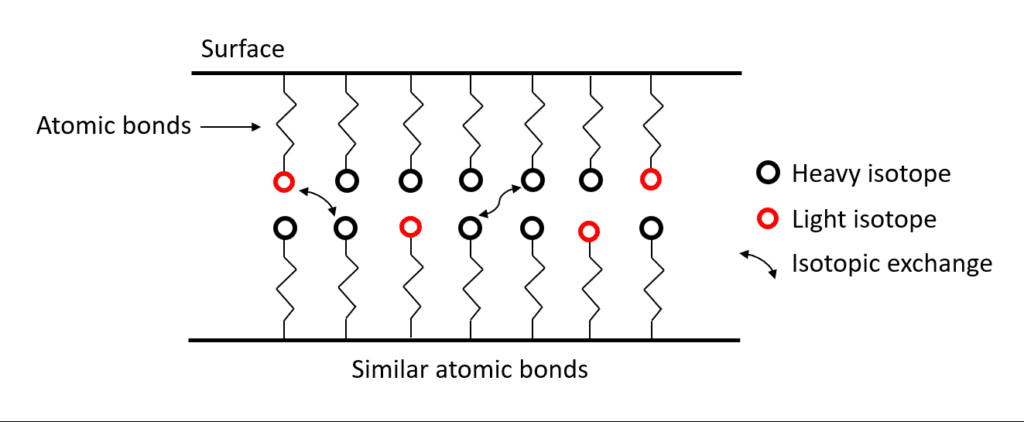
Representation of isotopic exchanges between two similar phases. The light isotopes are represented by the red circles while the heavy ones by the black circles. The two phases, which have similar atomic bonds, have the same concentrations of light and heavy isotopes, there is no isotopic fractionation between these phases.
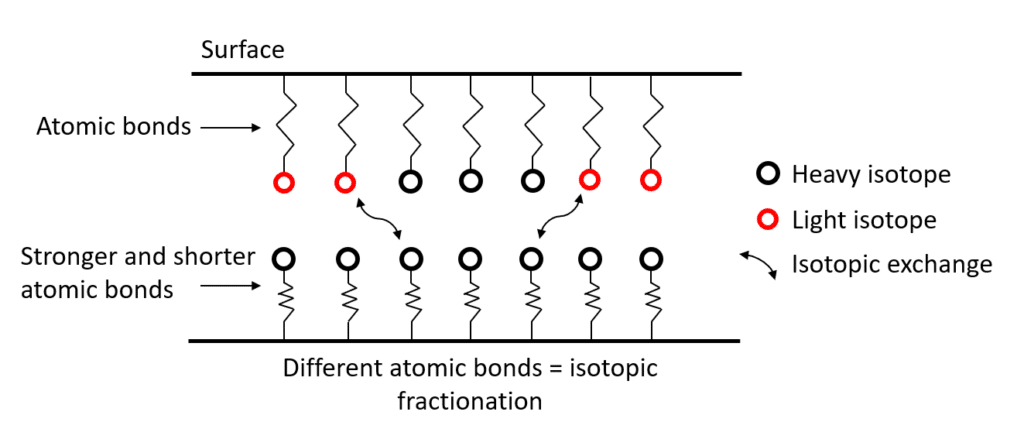
In this case, where the atomic bonds are not similar between the two phases, the isotopes will be distributed in a non-homogeneous way and change the isotopic signature of these two phases. This phenomenon is called isotopic fractionation.
In conclusion, all processes that affect atomic bonds are likely to cause mass isotopic fractionation by changing the distribution of light isotopes and heavy isotopes between different phases.
II. The different types of isotopic fractionations
There are two major types of isotopic mass-dependent fractionation: kinetic and equilibrium fractionation.
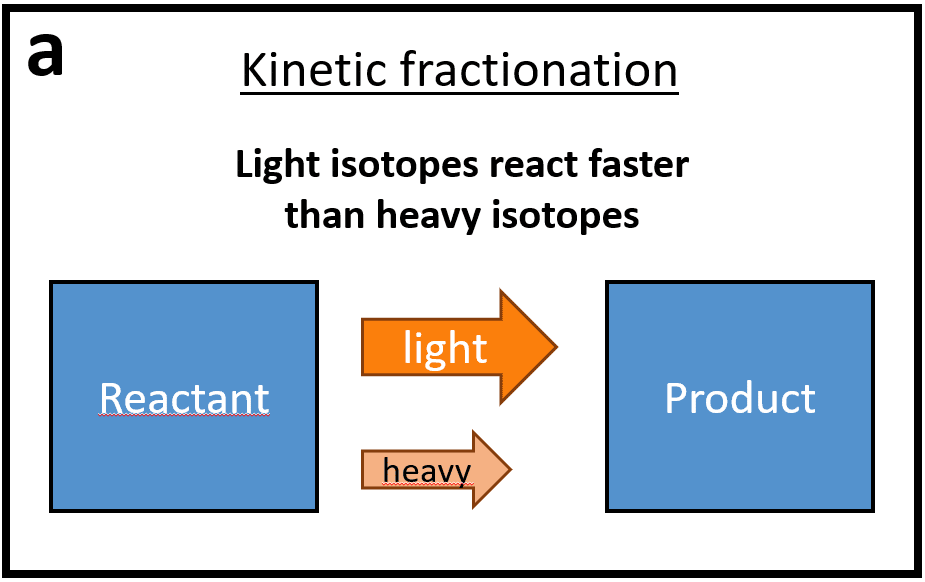
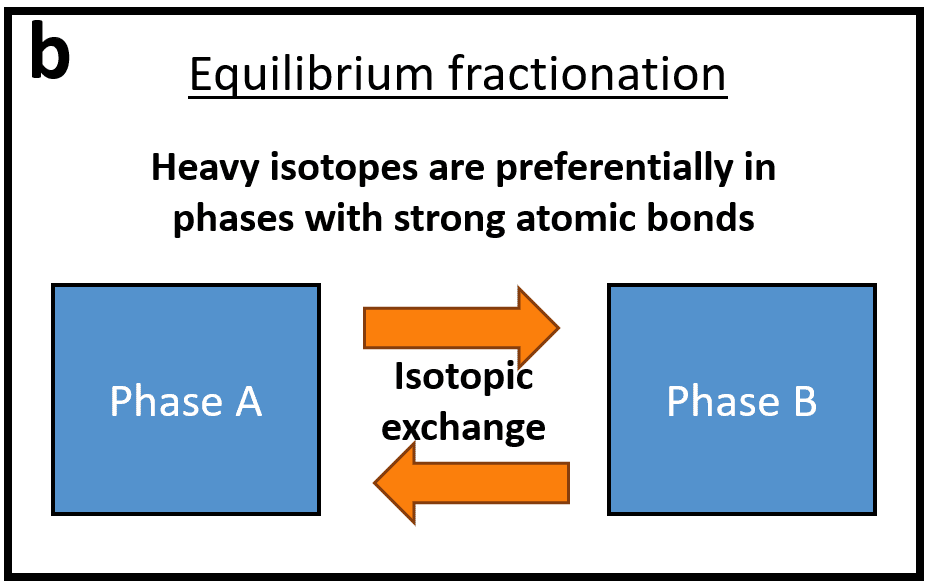
Schematic representation of the two types of isotopic fractionation: kinetic (a) and equilibrium (b). Adapted from Wiederhold 2015.
- Kinetic isotope fractionations are due to different reaction kinetics between heavy and light isotopes of an element that result from differences in the dissociation energies of the molecules they compose. The breaking of atomic bonds is easier for molecules containing light isotopes which have higher vibrational frequencies than those containing heavy isotopes, which leads to an enrichment in light isotopes in the product compared to the reagent. It is important to note that the kinetic fractionations are only visible and preserved as long as the reaction remains incomplete. Indeed, once the reaction is complete, and all the atoms have gone from reactants to products, the initial and final isotopic compositions are the same.
Equilibrium isotope fractionations are produced when two phases are in isotopic equilibrium with each other. They are due to the energy differences of the atomic bonds in which the isotopes are involved. Isotope equilibrium can be distinct from chemical equilibrium and depends only on temperature and not on pressure, which can be explained by the fact that isotopic substitution does not cause variation of the molar volume of the phases. Generally speaking, at isotopic equilibrium, heavy isotopes are enriched in environments with stronger atomic bonds (oxidized phases, shorter bonds…), which is explained thermodynamically by lower zero-point energies in molecules enriched in heavy isotopes compared to bonds with isotopes elements of the same element. It was shown that equilibrium isotope fractionation is not only a function of the relative mass difference between two isotopes or the strength of atomic bonds, but also of the type of bond. Covalent bonds, which are bonds exhibiting vigorous vibratory and rotational movements, are more strongly sensitive to mass differences and therefore will tend to generate greater isotopic fractionations, unlike metallic or ionic bonds, which are mainly influenced by electrostatic forces which depend on charge but very little on mass. This can be observed for the 12C and 13C isotopes of carbon, which, despite a much smaller relative difference in mass than between 40Ca and 48Ca, fractionate much more, due to their covalent bonds. The principles of quantum mechanics can be applied to predict the magnitude and direction of these fractionations at equilibrium.
III. General principles of isotope fractionation
Before going into more detail on each of the processes that fractionate metallic elements, general observations on the direction and amplitude of equilibrium isotopic fractionations are presented here, although they may vary from one isotopic system to another. They come from both theoretical and experimental studies.
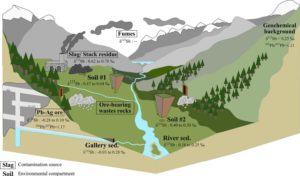
- Short atomic bonds are more robust and energetic and preferentially favor heavy isotopes; this is generally the case when the degree of coordination is low. For example, oxidized species will tend to be enriched in heavy isotopes compared to reduced species.
- The isotopic fractionations are generally higher for the light elements than for the heavy elements, since one neutron more or less causes a difference in mass, in proportion, greater.
- The isotopic fractionation linked to a given process decreases when the temperature increases, by a factor generally proportional to 1/T².
IV. How to study isotopic fractionation
Three complementary approaches can be implemented in order to understand isotopic fractionations and to be able to apply this knowledge to the tracing of sources of contamination in complex environments.
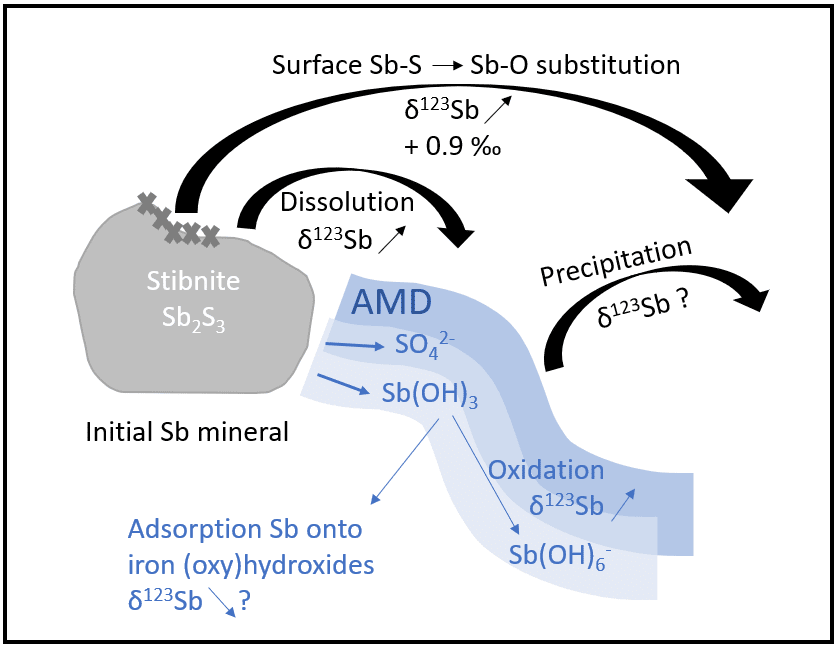
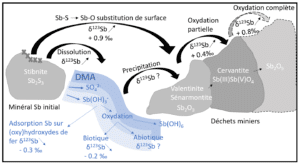
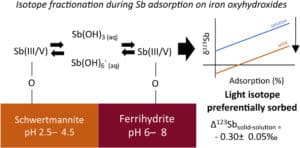
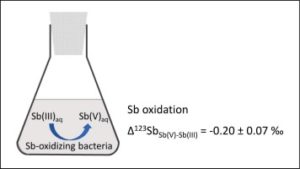
• Laboratory studies make it possible to reproduce physico-chemical processes in an isolated and controlled manner in order to quantify their impact on the isotopic fractionation of the targeted element. These experiments take place under controlled conditions and make it possible in particular to study equilibrium or kinetic fractionation during processes such as changes in the degree of oxidation, adsorption, precipitation, dissolution, etc.
• Theoretical studies of fractionation at equilibrium between two phases, make it possible to estimate the direction and the amplitudes of isotopic fractionations in a non-empirical way based on theoretical models of quantum mechanics. These splits are generally due to differences in energies associated with the vibrational frequencies of the atomic bonds as well as the type of bond. These theoretical models are based on equations from the beginning and middle of the last century (Schrödinger through his work on the time evolution of a non-relativistic mass particle, Born and Oppenheimer 1927 through their work resulting in adiabatic approximations, Urey, 1947 for the simplified equations of the ratios of the isotopic partition functions of molecules, and Kieffer 1982 for its extension to the condensed phases) but they have become popular with the rise of computing and means of calculation since the 1990s, and in particular with the density functional theory (DFT), which allows to have the best compromise between the precision of the results and the calculation times. These calculation methods require a good knowledge of the atomic environments of the elements in the minerals studied and take place most of the time in ideal cases (without impurities, with two or more phases in equilibrium). They make it possible to better understand and explain the isotopic fractionations observed during laboratory experiments or field studies.
• Field studies make it possible to measure the isotopic composition of natural samples. This composition derives from the different sources of the element as well as from the biogeochemical processes it undergoes during its transport. By crossing the isotopic data with other geochemical (concentrations, dissolved speciation) and mineralogical (solid speciation of the element) information, it is possible to identify the processes in which the element is involved and to evaluate factors of associated apparent isotopic fractionations.
Do not hesitate to help us improve our courses, all your feedback and constructive criticism are welcome, as well as your questions, in our forums or directly here, in comments!